Reckless driving?
Before too long, we’ll be able to encode unwanted organisms for extinction. But should we?
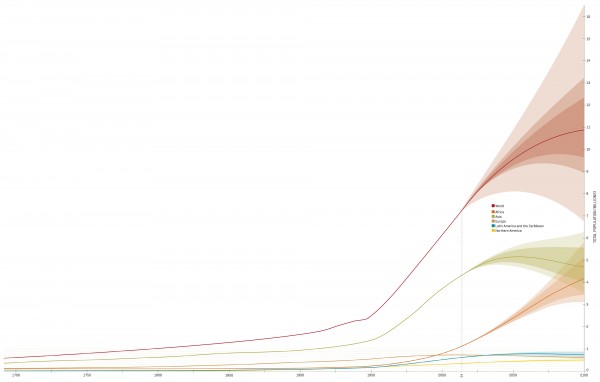
According to the World Health Organization, 214 million people contracted malaria in 2015. Around 438,000 never recovered. That’s the population of greater Wellington and a bit of the Kapiti Coast, wiped out by a single cell. Parasites of the genus Plasmodium infect certain species of equatorial mosquitoes, using them first as a nursery, and then as a hypodermic needle—the adult parasites are injected into their human hosts when a female Anopheles mosquito bites.
Researchers understood three decades ago that if the mosquitoes could be genetically re-engineered to repel Plasmodium, this vicious cycle could be broken. Isolating the right genes was tricky; creating transgenic mosquitoes was downright tough, but an even bigger problem remained: there was still no way to propagate those genes throughout wild mosquitoes. In 2003, Austin Burt, an evolutionary geneticist at Imperial College London, recognised that homing endonucleases—selfish enzymes that cut nucleotide chains in the middle, rather than acting at the ends—might be harnessed as Trojan Horses to speed the uptake of manipulated or engineered traits through a population at rates faster than normal.

But the endonucleases available at the time couldn’t deliver lasting, stable results. It was only in July 2014 that the Wyss Institute at Harvard announced a feasible alternative: using CRISPR-Cas9, an affordable genome-editing technology that can ‘snip’ target nucleotide chains and insert a new gene. It could theoretically sow almost any mutation from one chromosome to another, and on through sexually reproducing populations—a proposition scientists are calling a ‘gene drive’.
In sexual reproduction, the vast bulk of genes get a 50-50 shot at expression: you had an even chance of being made male or female. Genes that confer beneficial traits seek to immortalise the species, but CRISPR—or clustered regularly interspaced short palindromic repeats—exploits the existence of certain lone wolf ‘selfish genetic elements’. These egocentric bits of DNA or RNA exist solely to further themselves, even at the cost of the organism’s fitness. Selfish elements—also known as ‘jumping genes’—are present in all manner of creatures, including ourselves, and they prevail by breaking Mendel’s laws of inheritance. They’ve found a way to beat the 50-50 odds, pushing to the front of the inheritance queue.
By exploiting selfish elements, geneticists can now fit a gene drive to practically any DNA sequence (assuming they have a map of the target creature’s genome), effectively directing that species’ genetic future.
A change made to a given chromosome would copy itself into the DNA of every successive generation, rather than simply taking its chances.
There are two ways we might use gene drives to fight malaria: a British project—which includes Austin Burt—has engineered a suppression drive that would render all female offspring sterile. (Simply engineering sterile males hasn’t worked—somehow, females can tell, and avoid them.)
The ramifications are clear: it could drive Anopheles extinct, but even mosquitoes perform valuable services in an ecosystem, some of which we probably don’t yet understand. The other option is to accentuate the positive. Some mosquitoes already carry a gene that imparts resistance to the Plasmodium bacteria. When researchers used CRISPR to insert two Plasmodium-resistant genes into Anopheles mosquitoes, it worked. They passed those modified genes on to more than 98 per cent of their progeny.

If you’re inspired by the prospect, but disturbed by the notion, you’re in good company. Jane Goodall and David Suzuki are just two prominent commentators who have spoken out against “genocidal genes”. But researchers have no ambition to deploy their doomsday weapon anytime soon: as many have pointed out, there is no moral mandate to do so, and social licence will likely be hard to win. Furthermore, there’s no regulatory framework in place to manage gene drives: the whole point of them is to disperse far and wide, when current laws around genetically modified organisms (GMOs) are concerned entirely with keeping them out of the wild. The Cartagena Protocol on Biosafety tightly governs the transmission of GMOs across borders, but wild creatures don’t respect the lines on a map. It’s even possible that some malevolent entity may use gene drives as biological weapons to destroy crops and economies.
On the plus side, gene drives could save millions of human lives, alleviate the need for chemical pesticides and vertebrate toxins, immunise animals that host human diseases, wipe out pest plants and protect corals from bleaching. For New Zealanders, the possibilities are many. We could, for instance, eradicate bovine tuberculosis from carrier species such as possums and ferrets. Recently, the Government endorsed the notion of eliminating rats, possums and stoats from the country by 2050. Even with aerial 1080, cutting-edge autonomous traps and toxin stations, this is a big ask, but gene drives could greatly increase our chances of success, and they’re probably what the Government had in mind when it called for the “development of a scientific breakthrough capable of removing at least one small mammalian predator from New Zealand entirely.” A suppression gene introduced into a wild population might do that, exerting a bias that ensures all baby possums, rats, or stoats are born male, or infertile.
The question is whether we can keep native biodiversity safe while we use technologies such as gene drives—after all, they don’t call them ‘jumping genes’ for nothing.
We’re in a fortuitous position: we have only three species of native terrestrial mammals, and they’re all bats. Releasing gene drives into pest populations of mustelids, marsupials and rodents would, therefore, invite much less risk of collateral damage. Few other countries have that opportunity. (Many of Australia’s endangered native species, for instance, are mammals, and the danger of species-jumping there may be too great.)
Researchers have, unusually, formulated some safeguards against unexpected consequences even before gene-drive methodology has been refined. Aside from obvious containment precautions, some have proposed the simultaneous development of ‘reversal drives’, which could undo an earlier gene drive by inoculating a population against its effect. Another mechanism may be to first employ the CRISPR guide RNA without introducing the Cas9 ‘scissors’ enzyme, allowing researchers to test for the genetic alteration before committing the gene replacement into the wild population.
Any field trials are probably at least five years away, but progress in the labs will nevertheless outpace society’s ability to adjudicate on gene drive. It’s incumbent upon science and the Government to ensure people properly understand its risks and benefits before we unleash it.