Horizontal gene transfer
By taking a lateral approach, brainless blobs of plasma created the natural world.
On the morning of May 8, 2011, workers started calling in sick to a small organic farm on the outskirts of Bienenbüttel in northern Germany. By day’s end, a third of the farm’s staff was down with gastroenteritis. Over the next few days, distributors of the farm’s product—bean sprouts—started falling ill. Phone calls established that the farm’s seed supplier in Bordeaux, France, had staff away sick too.
They were eventually diagnosed with Escherichia coli infection, and by July 4, some 3000 Germans had been infected, and 53 were dead. The culprit was a coli bacterium, O104:H4. Investigators barely recognised it: until then, O104:H4 had been considered harmless. Now, suddenly, it was one of the biggest foodborne killers of all time.
When researchers probed its genome, they saw why O104:H4 had become a monster: it had somehow acquired deadly DNA from other E. coli strains.
Ordinarily, genes are transferred from parents to offspring in the traditional fashion—sexual or asexual reproduction—which biologists call ‘vertical’ gene transfer. But bacteria and some other single-celled creatures can also obtain DNA or RNA ‘horizontally’.
This happens in one of three different ways. In transformation, a cell takes up short fragments of genetic material from a separate strain. In transduction, DNA is carried from one bacterium to another by a virus. In bacterial conjugation, the transfer requires cell-to-cell contact—DNA is swapped in a parcel of genetic information called a plasmid.
But what turned O104:H4 rogue was a transposon, a mobile DNA element researchers call a ‘jumping gene’, which can gather up a resistance gene and insert it into a plasmid or chromosome. O104:H4 was the perfect storm in a cell; not only had it somehow acquired the genes to produce Shiga toxin—the pathogen responsible for dysentery—but it could now cluster inside the victim’s intestine, assembling itself into a structure not unlike a brick wall, secreting a thick protective shield of antibiotic-resistant mucus.

The regular form of Escherichia coli O104 is found in the intestine of most humans and other mammals. It has now acquired resistance to most contemporary antibiotics and has become one of the most virulent bacterial strains we know—a looming menace to human health. It usually infects people when they inadvertently eat food either incompletely cooked (E. coli is killed by sustained cooking above 70ºC) or contaminated by animal faeces. When it binds to the intestinal wall, the pathogen causes bloody diarrhoea, and can ultimately lead to kidney damage. What made the O104:H4 variant so deadly in Germany was that an unprecedented percentage of infections degenerated into this often-fatal stage.
Consider for a moment that many of the fundamental life processes on Earth—cell organisation, metabolisation, replication—were developed during the first two million years of life on the planet, before the advent of sexual reproduction. In other words, those early organisms had to do it without genetic evolution. It was long thought that these pioneering creatures could only ever bud off a clone of themselves, and that any variation must have been the consequence of subtle copying errors.
But as our understanding of horizontal gene transfer (HGT) widens, we can now assume that bacteria have been swapping DNA and RNA by a variety of other means for millennia. They didn’t have to wait for some random mutation to produce a beneficial trait (although that was, and still is, an important mechanism).
Once one strain picks up an advantageous gene from another, natural selection can get to work right away: that trait then gets handed down through lines both vertical and horizontal. This is how microbial life has been able to achieve so much with so little.
We now know that bacteria don’t have to be closely related to share genetic material. Horizontal transfer can occur across even distantly related bacterial and archaean strains, and even species—a bit like you patting a dog and acquiring some canine DNA in your genome. HGT opened the door to a vast trove of heritable information, and when that becomes an armoury, epidemiologists start to worry. When a bacterium finds a favourable environment, it gets straight to work, copying its DNA ready for release. That takes just 40 minutes. The cell doubles in size and starts dividing, roughly once every 20 minutes. But how, when it takes twice that long to replicate its DNA?
To swing this trick, the bacterium must turn itself into a high-speed Xerox—it starts the next copy before the previous duplicate is finished. This jump-start means at least one set of chromosomes will be ready before they get split. The rest will get finished in subsequent generations.
Bacteria can mutate on a dime, using material that, if it’s near enough, is plenty good enough. Once it’s acquired a trait, it can pass it on with breathtaking speed.
Research in 2012 found that certain E. coli and Klebsiella pneumoniae strains had acquired a whole new persistence and longevity—several weeks—on touch surfaces, including the stainless steel found in hospitals. We shouldn’t be surprised. When the discoverer of penicillin, Alexander Fleming, took the stage to receive his Nobel Prize in 1945, he warned his audience: “It is not difficult to make microbes resistant to penicillin in the laboratory by exposing them to concentrations not sufficient to kill them.” So it has proven. When his miracle drug was first trained on Staphylococcus aureus in 1943, resistance was unheard of. By 1950, 40 per cent of hospital S. aureus isolates were found to be penicillin-resistant. By 1960, it was 80 per cent.
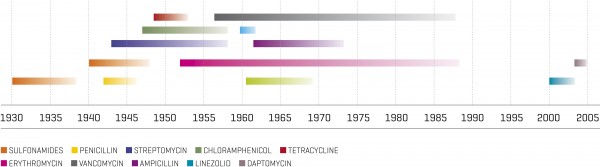
Penicillin’s successor, methicillin, enjoyed a similarly short career: within two years, resistant strains—now known as Methicillin-Resistant Staphylococcus aureus (MRSA)—appeared. In the early 1990s, a new hyper-resistant MRSA emerged, and this one attacked healthy people. In 2005, it killed nearly 20,000 Americans, more than HIV and tuberculosis combined.
In 1967, announcing the release of new antibiotics, U.S. Surgeon General William H. Stewart told health officials that we could now “close the book on infectious diseases”. Such is the folly of underestimating a tiny blob of plasma.